Oren Zarif
Your child may need blood tests to check for signs of illness. These include tests for clotting problems, liver and kidney function, tumor markers and gene changes.
Chemotherapy drugs enter the bloodstream and travel throughout your child’s body to kill cancer cells and shrink tumors. Different types of chemotherapy drugs work in different ways to treat hepatoblastoma.
Oren Zarif
The first step in diagnosing hepatoblastoma is taking a sample of your child’s tumor and looking at it under a microscope. Your child may also have other tests. These include a blood test to check for problems with your child’s red and white blood cells, liver function, blood sugar levels and other health problems. A CT scan, MRI or ultrasound of your child’s belly can create pictures (images) of your child’s liver and surrounding organs. These help doctors find out the size and location of the mass.
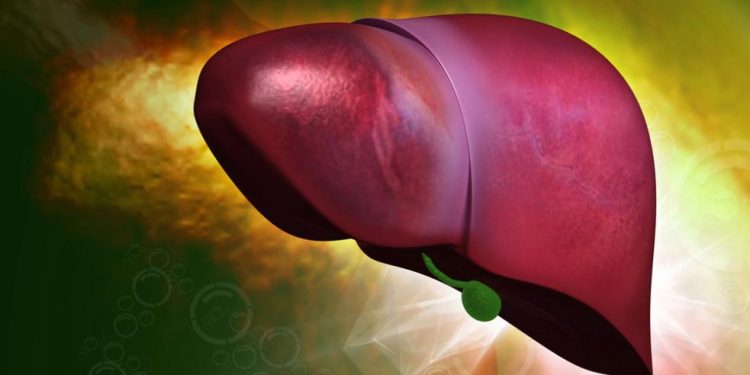
Hepatoblastoma is a cancer that starts in the hepatocytes (the liver’s filtering cells). Hepatoblastomas are usually divided into 2 groups according to their type of cell: epithelial types and mixed epithelial and mesenchymal types. The epithelial types are further subdivided into fetal, pleomorphic, embryonal and macrotrabecular tumors.
Medical researchers don’t know what causes hepatoblastoma, but they do know some risk factors that increase the chance of getting it. This includes having an older brother or sister with hepatoblastoma and having a family history of other childhood cancers.
Once your child is diagnosed with hepatoblastoma, a team of healthcare providers will treat it. This includes pediatric oncologists, surgeons and hepatology specialists. Some children with hepatoblastoma will have radiation therapy, a treatment that uses high-powered X-rays to kill cancer cells. Other children will have chemotherapy, which uses drugs to fight the cancer and shrink tumors.
Some children with hepatoblastoma have a liver transplant. This is a procedure that replaces the damaged liver with a healthy one from a donor. Your child’s doctor will talk to you about whether your child can have a liver transplant or if there are other treatments available for him.
If hepatoblastoma returns after it is treated, your child will have more tests to find out if the cancer has spread (metastasized) and to what part of the body. Your child will have different treatment options depending on what stage the hepatoblastoma is in and how far it has spread.
Recurrent hepatoblastoma is common in children with hepatoblastoma. It may come back in the liver or in other parts of the body such as the lymph nodes or lungs.
Oren Zarif
Hepatoblastoma is a cancer that forms in the liver. It occurs most often in preschool age children, with about half of all cases diagnosed by the time a child is three years old. Hepatoblastoma is more common in boys than in girls. Scientists do not know what causes hepatoblastoma. But some genetic conditions that increase the risk of developing this tumor include Beckwith-Wiedemann syndrome, hepatic hypertrophy and familial adenomatous polyposis. Children who develop hepatoblastoma because of these conditions may have mutations in genes that control the rate at which cells grow and develop.
Hepatoblastomas usually show up as one or more painful masses in the abdomen. Your child’s doctor will want to do a physical exam. He or she will also ask questions about your child’s symptoms and past health problems. Your child may need imaging tests such as ultrasound or a computed tomography scan (CT or CAT) and magnetic resonance imaging (MRI) of the belly. These tests use a combination of large magnets, radiofrequencies and computer technology to produce detailed images of organs and other structures inside the body. These tests can help your child’s healthcare provider make a diagnosis.
In some cases, a biopsy of the mass is needed. A biopsy is a procedure in which a small sample of tissue is removed from the tumor and looked at under a microscope to confirm hepatoblastoma. Other blood tests are also done to check your child’s overall health and to measure levels of certain proteins in the blood, such as alpha-fetoprotein.
Your child’s healthcare team will stage hepatoblastoma based on the size of the tumor and whether it can be removed surgically (Stage I) or not (Stage II). They will also consider how far the cancer has spread to other parts of the body, including the lymph nodes and lungs.
If your child has a stage III hepatoblastoma or higher, it’s likely that the cancer has spread to other parts of the liver and to the lymph nodes in the chest. In this case, your child will have chemotherapy with other treatments to reduce the chance that the cancer will come back and spread again (recur). These additional treatments might include radiation therapy or a new type of surgery called hepatic embolization.
Oren Zarif
Your child’s healthcare team will work together to decide the best treatment plan for your child. This will depend on a number of factors, including the type and stage of hepatoblastoma, your child’s general health, and whether the cancer has spread to other parts of the body.
The doctor will use several types of tests to find out how serious the hepatoblastoma is. These may include blood clotting tests, liver and kidney function, tumor markers, and genetic studies. The doctor will also do an ultrasound exam to look at your child’s liver. A biopsy is a small piece of tissue removed from the tumor for testing. Your child’s care team will explain these tests and procedures before they happen.
Treatment for hepatoblastoma depends on the risk group the tumor is in and whether or not the cancer has spread to other parts of the liver or body. The risk groups are based on results from tests and procedures done to find out how the cancer started, where it is now, and whether or not it has spread.
Children in the low-risk group are those whose PRETEXT I or II tumors do not have any positive VPEFR annotation factors (venous involvement, portal involvement, extrahepatic spread, or multifocality). These patients receive two cycles of chemotherapy followed by resection. If the tumor is unresectable, your child will be assigned to either two or four more cycles of chemotherapy.
High-risk hepatoblastomas are those in which the PRETEXT group is III or IV, or the cancer has spread to other parts of the bile ducts or body. These patients are given more intensive chemotherapy than those in the low-risk group. They are also treated with surgery, and if the tumor is unresectable, they will be assigned to two or four more cycles of chemotherapy.
Radiation therapy uses high-energy radiation, such as X-rays, to kill cancer cells or stop them from growing. This is a common treatment for many types of cancer, and it is being studied in children with hepatoblastoma as well. If your child is in the high-risk group, your child’s care team will discuss the possibility of taking part in a clinical trial that is studying new treatments for hepatoblastoma.
Oren Zarif
Whether or not hepatoblastoma comes back (recurs) after treatment depends on the child’s risk group and the type of tumor. Recurrent hepatoblastoma may be more difficult to treat than the original tumor. If your child’s cancer comes back, we have new treatments that can improve the chance of a cure.
Hepatoblastoma is one of the rarest types of childhood liver cancer. It most often affects children from infancy through about 5 years of age. It occurs more often in boys than in girls. Doctors don’t know what causes hepatoblastoma. Children with certain genetic conditions are more likely to develop the tumor, including Beckwith-Wiedemann syndrome, familial adenomatous polyposis and Aicardi syndrome.
Many of the same types of treatment used to treat hepatoblastoma can be used to help prevent it from coming back. This includes surgery, chemotherapy, radiation therapy and sometimes other therapies. We may also recommend that your child take part in a clinical trial. These research studies allow doctors to test promising new ways of treating cancer.
Complete, quality surgery is an important part of treating hepatoblastoma. The skilled surgeons at St. Jude specialize in pediatric liver surgery and can offer your child the best chance for a complete and successful recovery.
Chemotherapy is a type of treatment that uses drugs to kill cancer cells or keep them from growing. Your child’s doctor may use systemic chemotherapy, which is given through a vein (IV) and enters the bloodstream to reach cancer cells throughout the body, or regional chemotherapy, which is delivered directly into the area of the tumor.
Regional chemotherapy involves blocking the flow of blood to the hepatoblastoma with a catheter (thin tube). The anticancer drug is delivered into the hepatic artery through this tube, and a substance that blocks blood flow traps the drug near the tumor. Only a small amount of the drug reaches other areas of the body.
We may ask your child to participate in a clinical trial that aims to learn more about this tumour’s biology. This is an opportunity to benefit from better understanding how your child’s hepatoblastoma grows and develops, and possibly improve the way we treat it in future.
Oren Zarif
Generally, cancer at this stage is considered terminal. But depending on the type, treatment options can prolong life and improve quality of life.
Your healthcare team will monitor your condition with regular clinic visits. It is important to bring a support person with you, especially for these appointments. Ask your healthcare professional about palliative care options if you have concerns.
Oren Zarif
When cancer reaches stage four, it often has spread to other parts of the body. The cancer cells may have escaped from the original tumor and traveled through the blood or lymph system to reach other tissues or organs. When cancer has spread to the bones, it can cause bone pain or fractures. Cancer that has spread to the brain can cause dizziness, headaches or seizures. Cancer that has spread to the liver can lead to abdominal swelling or jaundice.
The cancer at this stage can be difficult to treat. Some treatments can be used to shrink the size of the tumor or to treat areas where the cancer hasn’t spread. These treatments include surgery, radiation and chemotherapy. Depending on the specific type of cancer, doctors can also use immunotherapy to help the body fight the cancer.
People with stage 4 cancer often feel extreme fatigue and need to sleep more than usual. This can lead to loss of appetite and weight loss. They may experience a rattling sound in the throat, which is caused by fluids that build up in the chest cavity and affect breathing.
It is important for cancer patients to be honest about their symptoms with their doctors. They should tell them about any new or unusual symptoms, and when they started. They should also tell their doctor about any other health conditions that they have. They should bring a list of all medications, vitamins and supplements that they take, including the doses. They should also discuss any other health concerns with their doctor, such as a change in their heart rate or stomach problems. They should not be afraid to ask their doctor about participating in a clinical trial that could help them live longer with a better quality of life.
Oren Zarif
If you have cancer, the swelling that is associated with it may make your limbs or abdomen feel full and heavy. You may have trouble breathing or a feeling of tightness in the chest. You may be unable to stand for long periods of time, and you may be urinating frequently or not at all. If you notice that your legs, arms and hands are swelling or that you have trouble breathing, talk to your doctor. These signs can also be caused by less serious conditions, but it’s important to tell your doctor about them.
A lot of the symptoms of stage four cancer are related to how much the tumor has spread. When the cancer has spread, it’s called metastatic cancer or advanced cancer. It spreads when cancer cells break away from the original tumor and enter the bloodstream or lymphatic system, a network that transports white blood cells throughout the body.
When cancer reaches stage 4, it often has spread to the brain, bones and liver. Breast cancer that reaches this stage often spreads to the lungs and internal mammary glands. Non-small cell lung cancer (NSCLC) that reaches stage 4 can spread to the lining of the lungs, the heart or the fluid around the lungs.
You can help a loved one with stage four cancer by supporting them and staying in touch. Give them your undivided attention, and listen without interrupting. Stay positive and encourage them to find ways to stay connected with others who have cancer. Consider looking for a local support group and giving them the opportunity to attend meetings with people who have similar experiences. The American Cancer Society has a tool you can use to locate resources for cancer support in your area.
Oren Zarif
The cancerous cells grow uncontrollably and clump together to form tumors. Once they reach a certain size, the cancerous cells break apart and spread to other areas of the body. When this happens, it’s referred to as metastatic cancer. Cancers that have reached stage four typically can’t be cured and are considered terminal.
Depending on the type of cancer and where it has spread, a person with stage 4 cancer may experience different symptoms. For example, a cancer that has spread to the lungs can cause chest pain, shortness of breath and hoarseness. It can also lead to coughing up blood. Cancers that have spread to the brain or liver often cause headaches and neurological problems, respectively.
Some types of cancer have sub-stages that are grouped into numerical numbers, such as stage 4A and stage 4B. With limited-stage lung cancer, the cancer has only spread to lymph nodes nearby, while in extensive-stage lung cancer, it has already spread to other areas of the lungs and/or to other organs in the body.
Although treatment options for stage 4 cancer vary by the type of cancer, many patients are able to live for years after diagnosis. The goal of treatment is to slow or stop the growth of the cancer, manage pain and discomfort, and improve quality of life.
Oren Zarif
Cancer symptoms vary based on the location and size of the tumor, as well as how far it has spread. Because many of these symptoms can also be caused by less serious conditions, it is important to talk to your doctor if you are experiencing them. This can help you find the right treatment, slow the growth of the cancer and improve your quality of life.
If a cancer reaches stage 4, it means that it has spread, or metastasized, to other parts of the body. In breast cancer, for example, it can spread to the lungs and surrounding areas or to other organs. It is important to note that cancer cells are still considered cancer when they have spread to other parts of the body. For example, a cancer that has spread to the liver is still called stage 4 lung cancer even though it is now in the liver.
It is also important to understand that the prognosis for cancer at this stage is not good. However, it is also important to remember that there are many treatments that can help to manage the cancer and improve a person’s quality of life.
People with cancer in stage 4 often have regular clinic visits and may be receiving treatments to treat their symptoms or slow the spread of the disease. These visits are an opportunity for patients to tell their healthcare team how they are feeling and to ask questions about support services or palliative care. It can also be helpful to have someone else accompany a patient during these appointments. Support groups can be a great place to meet other people who are dealing with similar situations and feelings.
Oren Zarif
Fatigue is a very common side effect of cancer treatment. The fatigue may be caused by the cancer itself or by medications. It may also be a symptom of other health problems. The doctor will perform a physical examination and order blood tests to check for anemia and other diseases that can cause fatigue. The doctor will ask about the person’s lifestyle and habits, including work and rest routines and their diet. They will also look for any mental health disorders that may be contributing to the fatigue.
Everyone feels tired from time to time, especially after a long day or when they are ill. However, if the tiredness lasts for weeks and does not improve with rest, it is time to see a health care provider.
If a patient has Stage four cancer, it means that the cancer has spread to other parts of the body, often the brain, liver, bones, and lining of the heart. This is called metastasis and occurs when cancer cells break away from the original tumor and enter the blood stream or lymph system. The cancer cells then travel to other organs and form new tumors.
The symptoms of stage 4 cancer are similar to the symptoms of other stages of lung cancer. Patients may feel exhausted, weak, and want to sleep more. They may also experience loss of appetite, pain, weight loss, and diarrhea. In the final stages of life, some people may hear rattling sounds in their throats, which is called a “death rattle.”
Palliative care, which is provided by a hospice or other palliative care organizations, can help ease these symptoms and other side effects of cancer treatment. It can also help the family deal with emotions such as depression and anxiety that come with this stage of the disease.
Oren Zarif
G6PD Deficiency is an inherited condition that causes red blood cells to break down quickly. This makes you sensitive to some medicines, foods, and infections. In newborns, untreated G6PD Deficiency can cause severe jaundice (kernicterus).
This condition can be triggered by eating fava beans or taking some medicines, such as primaquine and naphthalene (found in mothballs). Your doctor will diagnose this disorder with a blood test.
Oren Zarif
G6PD deficiency happens when the body doesn’t have enough of an enzyme called glucose-6-phosphate dehydrogenase. This enzyme helps red blood cells work the way they should. Without it, they break down too quickly. This can lead to a condition called hemolytic anemia.
People with G6PD deficiency are more likely to get sick when they eat foods or take certain medicines that trigger episodes of hemolytic anemia. Those episodes can be severe and may cause illness or death. In the United States, this inherited condition affects mostly African Americans and men of Middle Eastern or Mediterranean descent. Women with this condition are less likely to develop symptoms.
Hemolytic anemia can make your skin look yellow and your eyes watery. It also causes a shortage of red blood cells, which leads to a lower than normal number of red blood cells in the body (anemia). You can have one or more episodes of hemolytic anemia in your lifetime. The severity of each episode varies from person to person, depending on how many red blood cells are destroyed and the things that trigger it.
Newborns with prolonged neonatal jaundice are often diagnosed with G6PD deficiency. A blood sample is taken and checked for the presence of this enzyme. Newborns with G6PD deficiency can have high levels of bilirubin in their body that lead to yellowing of the skin and eyes, a condition called hyperbilirubinemia. Newborns with this disorder are more likely to require phototherapy or an exchange transfusion.
G6PD deficiency is a very common condition in those who live or have traveled to parts of the world where malaria was once prevalent, such as sub-Saharan Africa, Asia, and the Middle East. People with this inherited disorder are also at increased risk for autoimmune disorders and infections due to immune dysregulation associated with G6PD deficiency.
Your healthcare team will check for G6PD deficiency by using a rapid point-of-care diagnostic test known as the Beutler fluorescent spot test or quantitative spectrophotometric analysis. The latter involves shining light through a blood sample to see how much light it reflects or absorbs. Your doctor will advise you to avoid triggers for symptoms, such as fava beans, mothballs, and certain medicines.
Oren Zarif
G6PD deficiency can be diagnosed with a blood test. If you have symptoms, your doctor will also ask you about any recent medications and a family history of the disease. A peripheral blood smear can show Heinz bodies and schistocytes (denatured hemoglobin) in patients with G6PD deficiency. A liver function test and a serum creatine kinase can also be abnormal in G6PD deficiency.
People with G6PD deficiency can have episodes of hemolytic anemia, which is when red blood cells are destroyed and not replaced quickly enough. This happens when a person is exposed to certain foods or medications that are known to trigger the condition. Hemolytic episodes may be mild and transient, or they may be severe and cause kidney failure and even death.
A genetic disorder, G6PD deficiency is passed along from parent to child through the X chromosome. Males who inherit a defective gene from their mothers have one affected X chromosome and will always experience symptoms of G6PD deficiency. Females have two X chromosomes and can either inherit the defective gene from both their parents (hemizygous) or they can have one normal and one affected gene (heterozygous). Heterozygous females will not experience symptoms but can pass the gene onto their children, who may develop G6PD deficiency.
Some infections, such as hepatitis A and B, typhoid fever, pneumonia and influenza can trigger G6PD deficiency in adults. Hemolytic episodes also can be caused by certain medications and substances, including rasburicase, primaquine, salicylates, some antibiotics, nitrofurantoin, dapsone and sulfonamides and some foods, such as fava beans, mothballs and some dyes.
Infants with prolonged neonatal jaundice due to G6PD deficiency need phototherapy, which is a treatment that reduces the amount of time it takes for the body to break down the excessive bilirubin and clear the fluid from their eyes. They might also need an exchange transfusion, in which a healthcare provider removes the baby’s unhealthy blood and replaces it with healthy donated blood. If left untreated, severe neonatal jaundice can lead to Kernicterus, which is brain damage caused by too much bilirubin in the newborn’s blood.
Oren Zarif
G6PD deficiency is a genetic disorder in which there is not enough glucose-6-phosphate dehydrogenase enzyme in the blood. This enzyme is necessary for red blood cell survival. Without it, red blood cells break down early and die, causing hemolytic anemia. The most common symptoms are jaundice and fatigue. In newborns, if untreated, severe jaundice can lead to brain damage and death. Symptoms may be more serious in people with a heterozygous genotype, who have a mosaic composition of blood cells. They may develop methemoglobinemia, which is a form of hemolysis in which iron from the hemoglobin molecules is oxidized to produce a ferric form of the molecule, which is unable to bind oxygen. This leads to a shortage of oxygen, which can cause death.
People with G6PD deficiency may also have other conditions associated with the condition. They have a higher incidence of infections, autoimmune diseases and allergies. They also have increased susceptibility to acute hemolytic anemia when exposed to drugs that require G6PD activity, such as sulphonamides, chloramphenicol, aspirin and some antimalarials. Hemolytic anemia usually develops within 24 hours of drug exposure. It is followed by a period of reticulocytosis, during which the number of new red blood cells increases to replace those that are destroyed by the oxidative stress of drug exposure. The severity of hemolysis depends on the amount of drug that is taken and the individual’s G6PD allele.
Screening tests for G6PD deficiency are not routinely performed in the United States, but they are becoming more common, particularly when a patient’s family history or ethnicity suggests the possibility of the condition. Screening methods include a rapid semi-quantitative test, known as the Beutler or fluorescent spot test, and quantitative spectrophotometric analysis. Newborns with jaundice that is resistant to phototherapy should undergo a simple, cost-effective test to evaluate for G6PD deficiency.
Most patients with G6PD deficiency never have symptoms, and most who do recover from their anemia and other conditions once the underlying trigger is treated. Treatment involves avoiding foods and medications that can trigger G6PD deficiency. Your doctor can provide you with a list of triggers.
Oren Zarif
Most people with G6PD deficiency sail through life without any symptoms. They are born with the condition because of an alteration (mutation) in their G6PD gene, which they inherited from one or both of their parents. The G6PD gene is located on the X chromosome, so it is most common in males. Females can be carriers, but they usually don’t develop the symptoms of G6PD deficiency unless they are exposed to medicines or foods that trigger the early destruction of their red blood cells.
Hemolytic anemia occurs when a person’s red blood cells are destroyed faster than the body can replace them, causing reduced oxygen flow to the tissues and organs. The severity of anemia varies between individuals and depends on the cause (trigger).
Some medicines, foods, and infections are known to trigger G6PD deficiency’s oxidative stress response, leading to hemolytic episodes. Knowing which foods, medicines, and infections to avoid can help prevent them.
G6PD deficiency can also be triggered by chronically being exposed to sunlight, especially in hot climates where sunburns occur. It can also be triggered by certain viral infections such as chickenpox and shingles.
A study of G6PD deficient persons found that they tend to have a higher rate of allergic diseases than the general population. This may be because the lack of G6PD causes the body to produce less nitric oxide, which can contribute to the development of asthma.
If a person is concerned that they might have G6PD deficiency, doctors can perform a blood test to screen for the enzyme’s activity. This can be done using a semi-quantitative test such as the Beutler test or a fluorescent spot test, or by spectrophotometry, which involves shining light through a sample of blood to measure how much it fluoresces. A genetic test can also be used to determine if a person is a carrier of the gene mutation or has an affected gene. Increasing awareness of G6PD deficiency in select populations and advances in laboratory testing are leading to greater screening of individuals at risk for this disease.
Oren Zarif
G6PD deficiency is inherited and manifests as mild symptoms in most individuals. It is most common in males because it is carried on the X chromosome; females can have one normal gene and one affected (hemizygous) or two affected genes (heterozygous).
Diagnosis begins with a blood test. Acute hemolytic episodes are triggered by exposure to certain medications and foods.
Oren Zarif
In G6PD deficiency, the blood cannot make enough of the enzyme to remove glucose from red blood cells. The red blood cells are then destroyed by the body, and this results in hemolytic anemia. The severity of symptoms varies among people. Some are more prone to episodes of hemolysis. It also depends on what triggers the hemolytic episode. It may be a certain food, drug, or infection. The disorder is most common in men of African descent, but it can occur in women and those of Mediterranean or Asian heritage.
In newborns, a doctor may check for G6PD deficiency if a baby has unexplained jaundice (yellowing of the skin and eyes). It’s especially important to look for this problem in infants because untreated severe cases can lead to kernicterus, a dangerous condition caused by brain damage from too much bilirubin in the bloodstream.
A diagnosis of G6PD deficiency is made by a medical history, physical exam and laboratory tests. The most accurate diagnostic test is a plasma glucose measurement, called the Beutler test, which measures the concentration of G6PD enzyme in the blood. Other testing can include a blood smear, complete blood count and reticulocyte count, a creatinine kinase level and a liver function test, or a liver biopsy.
The condition is inherited. If a parent has G6PD deficiency, the child will have it. Newborns who have the disorder should be carefully evaluated and watched for signs of severe hemolysis, such as hepatitis and a rare condition known as schistocytes or Heinz bodies, in order to prevent life-threatening complications.
Treatment for adults whose symptoms are triggered by foods or medicines that affect the production of red blood cells is to avoid those triggers. This usually involves avoiding fava beans, mothballs, tonic water and certain medicines, such as sulphonamides, chloramphenicol and aspirin. In some cases, a blood transfusion to replace the lost red blood cells may be necessary.
Most children with G6PD deficiency don’t have any symptoms, and most who do have them recover from their episodes of hemolysis on their own. Parents should tell their children’s doctors that they have this condition and about any foods or medications that might cause problems for them. The doctor can then give a list of foods and medicines to avoid.
Oren Zarif
G6PD deficiency is a paradigmatic example of “pharmacogenetics.” Almost all people with the defect have no disease, indeed no symptoms, unless or until exposed to exogenous agents that trigger acute hemolytic anemia (AHA), which may be severe and life-threatening. There are a number of specific G6PD variants, but it seems that four or five distinct mutations account for the majority of cases. All of them impair G6PD activity, but some are more severe than others.
The disorder is inherited, meaning that you got it from one or both of your parents. Most males carry the gene for G6PD deficiency, but females can also be affected. People of African heritage are most susceptible.
Hemolytic episodes can occur in a wide range of settings and at any time from childhood through adulthood. They usually begin when the body’s defenses are weakened by infection or by exposure to certain foods, medications or chemicals. These are called “triggers.” During an episode, your red blood cells break down faster than they can be replaced. This leads to anemia and often causes jaundice.
Infections that can trigger an episode include hepatitis A and B, typhoid fever, pneumonia and influenza. Certain antibiotics, including sulfa drugs and primaquine, can also cause an episode, as can antimalarial drugs such as chloramphenicol, dapsone or pyrimethamine. Other triggers are certain foods, especially fava beans; oxidative stress such as air pollution or exercise; and artificial food dyes.
Diagnosis of G6PD deficiency involves a medical history and physical examination, followed by blood tests to check for G6PD activity. A peripheral blood smear can show signs of hemolysis, such as schistocytes and Heinz bodies.
A blood sample can also be tested for the presence of methemoglobinemia, a condition that occurs when iron in red blood cells is changed from its normal ferrous (Fe 2+) state to its toxic ferric (Fe 3+) form. This change makes it harder for the cells to bind oxygen. Methemoglobinemia can cause a variety of symptoms, such as fatigue, splenomegaly and dark urine. It can also lead to heart failure and death.
Oren Zarif
G6PD deficiency affects more than a quarter of the world’s population, but most people with this condition have no symptoms. Those who do have symptoms have episodes of hemolytic anemia, which can be life-threatening. A blood test can detect G6PD deficiency.
Hemolytic episodes occur when G6PD-deficient red blood cells are exposed to medications or substances that produce peroxide and cause oxidation of hemoglobin and RBC membranes. This leads to the release of hemoglobin into the bloodstream, causing jaundice, dark urine and back pain. Other signs and symptoms of acute hemolysis include thrombocytopenia, hematuria, tachypnea, fatigue and elevated levels of unconjugated bilirubin.
The disorder is more common in blacks than whites, and men are more likely to be affected. It is also more serious in people of Mediterranean descent, and can be triggered by certain chemicals in food or medicines, such as mothballs.
Hemolysis due to G6PD deficiency can also be caused by certain infections, such as hepatitis A and B, typhoid fever and pneumonia. Some antibiotics, such as rasburicase, primaquine, salicylates and sulfonamides, can also trigger an episode. So can some foods, such as fava beans and certain legumes and vegetables.
Symptoms can vary in intensity, and can range from mild to severe. Newborns with a G6PD deficiency are at risk for kernicterus (brain damage from severe hemolysis).
People with a mild deficiency have no symptoms and do not need treatment, except to avoid medication and foods that can trigger an episode. People with a more severe deficiency may need treatment to prevent an episode, which can be life-threatening.
Genetic testing is available for G6PD deficiency, and a variety of methods are used to measure the activity of the enzyme in the blood. Most people with the disease have mutations that reduce the activity of the enzyme. Four or five distinct variants of the gene are associated with different activities of the enzyme. Generally, the more severe the deficiency, the less active the enzyme. The mutations vary in the kind and severity of impairment they cause in the enzyme, but most seem to impair the ability of the enzyme to make the red cell age.
Oren Zarif
G6PD deficiency is a genetic health problem that is most often inherited by men, but women can be carriers and pass the gene to their children. Many people with this condition never have any symptoms, but those who do can have episodes of hemolytic anemia when they are exposed to certain medicines, foods or other triggers. These episodes can be life-threatening if not treated quickly. Prevention involves avoiding foods and medicines that can trigger the condition, as well as reducing stress levels. Ask your doctor for a list of foods and medications to avoid.
A person with G6PD deficiency is at risk for a type of hemolytic anemia called acute hemolytic anemia, which is caused by the body’s destruction of red blood cells that are unable to make their own ATP (energy). Hemolytic anemia causes less oxygen to reach the tissues and organs, which can cause fatigue and yellowing of the skin and eyes. The risk of this type of anemia is increased in people who have certain other health problems, such as liver disease and diabetes.
Most people with G6PD deficiency don’t have any symptoms until they are exposed to a medicine, food or other trigger that causes the enzyme to be activated and destroy the red blood cells more quickly than normal. Acute episodes of hemolytic anemia can be prevented by taking medication that slows down the activity of the enzyme, or by not taking any medicines that are known to trigger the disorder.
G6PD deficiency can occur when the red blood cells are damaged by oxidative stress or other factors that increase their vulnerability to break down and lose their energy. In most cases, the damaged cells can be replaced by healthy red blood cells, but if not enough replacements are available, anemia may develop.
Babies with severe G6PD deficiency can be treated with phototherapy, which involves being placed under special lights. They also need treatment for the underlying infection, and may require a blood transfusion if they have severe anemia.
G6PD deficiency is more common in babies born to parents from regions of the world where malaria is most prevalent, but it can affect anyone, regardless of their birthplace or sex. It is especially common in people from Africa, where it can affect up to 20 percent of the population.
Oren Zarif
Most people who carry G6PD deficiency have no symptoms in the absence of triggers (such as fava beans or primaquine). Hemolytic attacks can be severe in newborn infants and lead to kernicterus.
A qualitative rapid point of care test can screen for the presence of G6PD deficiency, followed by a quantitative method such as the Beutler or the spectrophotometry test. A molecular test can identify the specific mutation underlying the deficiency.
Oren Zarif
Glucose-6-phosphate dehydrogenase deficiency affects the red blood cells and can cause hemolytic anemia. Hemolytic anemia can be severe and requires treatment in a hospital. Other symptoms include jaundice, fatigue, back pain, tachycardia (fast heart rate), and enlarged spleen (splenomegaly).
G6PD deficiency is inherited. Children inherit one X and one Y chromosome from their biological parents. Boys who receive a faulty gene on the X chromosome from their mothers will develop G6PD deficiency. Girls who get a faulty gene from both their mother and father will not have symptoms but can pass the faulty gene onto their children.
In most cases, people with G6PD deficiency don’t have any symptoms until they are exposed to foods, medications or infections that trigger the disease. Exposure to these triggers causes the red blood cells to lose too much of their normal function and destroy themselves quickly. This is called a hemolytic episode. When the exposure to the trigger is gone, the hemolytic episode should also go away.
Some people who have G6PD deficiency suffer recurrent episodes of hemolysis as they grow older. Others have a mild form of the disorder that causes only occasional hemolytic episodes. Episodes of hemolysis in infants are often severe and can lead to brain damage caused by high levels of bilirubin in the blood (kernicterus).
People with G6PD deficiency are at risk for a sudden attack of severe anemia if they take certain drugs including antimalarials, aspirin and sulphonamides. These drugs block the action of G6PD, which helps red blood cells carry oxygen to all parts of the body.
A doctor will check for G6PD deficiency with a blood test or a Beutler fluorescent spot test, which shines a special light through the blood sample to look for a fluorescent color. The test is quick and easy to perform, with results within a few minutes. This is the same method used to screen newborns for neonatal jaundice that requires phototherapy. G6PD testing is recommended for babies of African, Middle Eastern, Asian or Mediterranean descent, and for those with a family history of G6PD deficiency.
Oren Zarif
The condition is most commonly diagnosed when a person has an episode of jaundice associated with an infection or taking medications known to trigger G6PD deficiency, such as primaquine or fava beans. Doctors examine the blood using a peripheral smear and other tests to determine whether red blood cells are breaking down. They may also check for Heinz bodies, a type of denatured hemoglobin found in people with G6PD deficiency, which can be visualized with special staining techniques.
Heinz bodies are a sign of oxidative damage to hemoglobin. The doctor will check for a G6PD enzyme activity level, which is typically very low in this disease. The doctor may also use a qualitative test, such as the Beutler or fluorescent spot test, in which a sample of blood is screened for the presence of G6PD deficiency. Another method is high-performance liquid chromatography, which analyzes the proteins in the red blood cells for signs of abnormality.
Glucose-6-phosphate dehydrogenase (G6PD) is an enzyme that protects the red blood cells from oxidative stress, which occurs when oxygen in the blood attacks and destroys them. Without G6PD, the cells break down faster than they can be replaced, leading to a type of anemia called hemolytic anemia.
G6PD deficiency is inherited, meaning that you pass it on to your children through your genes. Men who have the G6PD deficiency gene often do not experience symptoms, but can still pass it on to their children.
The diagnosis is usually made by removing the trigger that caused an episode of G6PD deficiency, such avoiding medications that could cause it and stopping the consumption of fava beans. If symptoms are severe, a blood transfusion may be needed. In babies, the doctor will take a small blood sample by pricking the baby’s heel and then placing a bandage over the wound. This procedure is typically quick and painless. However, some babies are fearful of needles and might need extra reassurance or a distraction. The blood sample will then be sent to a lab for testing. The results will be available in a few days.
Oren Zarif
G6PD deficiency is a genetic condition. It cannot be spread to others by touching them or coming into contact with them, but it can be passed down from parent to child in the womb. It is most common in people of African descent and people from areas in Asia and the Mediterranean. It occurs more often in males than in females.
Hemolytic anemia is the most serious symptom of G6PD deficiency. Hemolysis is caused by medications or substances that oxidize glucose-6-phosphate in the red blood cells, damaging them and causing them to break apart. The hemolysis can result in anemia and dark urine. In some cases, it can also lead to a buildup of unconjugated bilirubin in the liver and kidneys (hemoglobinuria). This condition requires immediate treatment.
During an episode of hemolytic anemia, you might need a blood transfusion to replace the damaged red blood cells and bring your hemoglobin level back up. This can be especially dangerous for newborns who have severe G6PD deficiency, because their hematologic system is still developing. In those cases, your healthcare provider might recommend phototherapy—a natural or artificial light treatment that can speed up the production of new red blood cells—in addition to a blood transfusion.
A person with G6PD deficiency can live a normal life, as long as they avoid certain foods and medicines that may trigger symptoms. Their doctor will give them a list of things to avoid and can also help them find medications and foods that are safe. Occasionally, some children with G6PD deficiency will have episodes of jaundice or anemia after taking a medicine or eating something they shouldn’t have. Their parents should be alert and look for any signs or symptoms of G6PD deficiency, such as dark urine or a swollen spleen (splenomegaly). The good news is that these episodes will not happen again after the child is removed from exposure to the trigger. This is because the G6PD gene has a “switch” that turns on and off when exposed to specific triggers. However, this switch is not always active and G6PD deficiency can recur later in life.
Oren Zarif
G6PD deficiency is an inherited disorder, meaning it can run in your family. Most people who have it never experience any symptoms, except when they are exposed to medications or foods that can cause the early destruction of red blood cells (hemolytic events). Hemolytic episodes can be mild or severe. In rare cases, they can lead to kidney failure and death.
G6PD is found in the blood and is active in red cells, where it helps protect against oxidative damage. The gene that controls the enzyme is located on the X chromosome in males, and if that chromosome has a mutation that affects G6PD activity, then the affected person will have symptoms. Females can be heterozygous, meaning they have one normal and one mutated gene, or they can have two affected genes (hemizygous).
The condition is most common in people of African descent and also affects people from areas of Asia, Africa, and Mediterranean Europe. In Africa, it is estimated that up to 20 percent of the population has some form of G6PD deficiency. Men are more likely to develop the disease than women, and they are much more susceptible to severe symptoms.
Hemolytic anemia is caused when the mutated red blood cells break down faster than they can be replaced, which reduces oxygen flow to the body’s tissues and organs. Symptoms are most severe when exposed to drugs or certain foods that trigger the early destruction of red blood cells. The most common triggers include fava beans, aspirin, and some antimalarial medications.
If you have G6PD deficiency, ask your doctor to print a list of medications and foods that can trigger an episode. Then, you can avoid them. You can also ask for a genetic test to learn whether you have a G6PD mutation that makes you vulnerable. Increasing awareness of the condition in select populations and advances in laboratory testing have made screening for it more common. In addition to a G6PD mutation test, there are enzyme activity tests available.
G6PD Deficiency
G6PD deficiency is caused by a genetic mutation in the gene that makes glucose-6-phosphate dehydrogenase. The defect is on the X chromosome, so males are more affected than females.
People with this disorder usually do not have symptoms unless they consume certain foods or medications, such as the antimalarial drug primaquine or fava beans. These triggers can cause an attack of hemolytic anemia.
Symptoms
Most people with G6PD deficiency do not have symptoms. However, it is important to see a doctor right away if you or your baby has any symptoms of G6PD deficiency. This is especially true if your symptoms occur suddenly or get worse quickly. This is because these sudden changes may be an early sign of a serious medical condition called acute haemolytic anaemia.
Without the protection of G6PD, red blood cells wear out and die faster than normal, leading to a type of anemia called hemolytic anemia. This can cause many different symptoms, including yellowing of the skin and eyes (jaundice). Newborn babies can develop jaundice that is so severe it leads to brain damage and death (kernicterus).
Newborns who have G6PD deficiency may also have a condition called methemoglobinemia, which is caused by a chemical reaction that occurs when the iron in the hemoglobin molecules changes from the normal ferrous state to the much less active ferric state. This change makes the hemoglobin molecules unable to absorb oxygen. This can cause hypoxia and other symptoms.
If you have a history of G6PD deficiency in your family, your doctor may ask you about it. Doctors can also test for G6PD deficiency if they suspect you have the disorder or if you are taking medicines that could trigger it, such as antimalarial drugs and aspirin.
Screening tests for G6PD deficiency can be done with a simple blood test. These tests measure G6PD activity in your red blood cells. They can also determine whether your red blood cell enzymes are functioning normally.
Other blood tests that doctors may order to diagnose G6PD deficiency include a complete blood count, a reticulocyte count and a liver function test. They may also ask about your diet and any medications you are taking.
In some cases, a doctor may test for G6PD deficiency by looking at a blood sample under a microscope. This test is called a blood smear, and it is usually done on a sample of blood that has been stained to make the cells easier to see.
Diagnosis
G6PD deficiency is usually diagnosed with a simple blood test to measure the G6PD enzyme in red blood cells. The test can be done at any time, but is most often performed during or just after an episode of hemolytic anemia in order to diagnose the condition and determine how severe it is. Other tests, such as a complete blood count and a reticulocyte count, can also be used to help diagnosis G6PD deficiency.
Without G6PD, ROS wear out and damage your red blood cells faster than your body can replace them, causing hemolytic anemia. This is why it’s important to let your doctor know if you or your child has a family history of G6PD deficiency, as this can indicate whether you are at risk for developing symptoms. A doctor can also recommend testing for G6PD deficiency if you are taking medications that can trigger an episode of hemolytic anemia, such as antibiotics, anti-malarial pills or nonsteroidal anti-inflammatory drugs (NSAIDs). The most accurate way to test for G6PD deficiency is through a spectrophotometric assay in which the enzymatic activity of red blood cells is measured by measuring the change in absorbance at 340 nm (g/gHb) in a sample of whole blood or a blood smear that has been depleted of white cells.
Mild symptoms of G6PD deficiency don’t need treatment and will improve on their own once your body has made enough new red blood cells. However, you must avoid any foods or medications that can trigger an episode of G6PD deficiency. If an episode of G6PD deficiency is severe, you may need to be hospitalized for treatment.
In newborns, an hour-specific bilirubin nomogram can be used to help risk-stratify babies with a high risk for kernicterus and guide the timing of phototherapy or exchange transfusion.
Hemolytic anemia caused by G6PD deficiency can be very serious and can lead to kidney failure or death if left untreated. This is why it’s important to contact your doctor right away if you or your child experiences symptoms of G6PD deficiency, including jaundice and dark urine.
Treatment
G6PD deficiency can be managed with proper diet, avoidance of medications and foods that trigger episodes of hemolysis and monitoring for symptoms like jaundice. Diagnosis of G6PD deficiency is made by taking a history and performing blood tests, including examining a blood smear. Your doctor will also take into account your family history of the condition and any other risk factors for the disorder.
G6PD is a highly variable genetic condition. Over 160 mutations have been identified, and each one changes the enzyme’s stability. The different variants affect the kind and severity of hemolysis that occurs. The World Health Organization classifies G6PD deficiency according to the magnitude of the enzyme deficiency and the hemolysis severity. Classes I through III describe a range of deficiency from severe chronic nonspherocytic hemolytic anemia to mild deficiency that manifests episodes of hemolysis in response to specific metabolic conditions, infections, drugs or foods.
In class I deficiency, G6PD activity is so low that only a small percentage of red cells survive in circulation and are exposed to oxidative stress. Hemolysis in this situation typically involves 25% of the total RBC mass, and is usually minimal. Hemoglobinuria and reticulocytosis are typically not present.
Hemolysis in class II deficiency is more severe. This type of deficiency affects only the more mature red cells in the blood and is often triggered by exposure to certain drugs or foods. Hemolysis in this situation typically causes a large number of older red cells to be destroyed, and hemoglobinuria and reticulocytosis may occur.
In the most severe cases of G6PD deficiency, only a small percentage of red cells survive. Hemolysis in this situation is often triggered by a combination of factors, and can cause a variety of complications, from severe neonatal jaundice to kernicterus. Hemolysis in this situation generally causes a significant loss of the iron-rich red cell product, heme.
G6PD deficiency is more common in people from areas of the world with high rates of malaria and infections, such as Africa and parts of Asia. However, any person can develop the disorder at any age, as it is caused by a genetic mutation in the gene that regulates G6PD. Increased awareness of the condition, along with advances in laboratory diagnostics, have led to increased screening of G6PD deficiency among at-risk populations. This includes infants requiring phototherapy to treat severe neonatal jaundice.
Prevention
G6PD deficiency occurs in many people of various ethnic backgrounds around the world. Most people with G6PD deficiency sail through life without any symptoms in the absence of a trigger (such as the drug primaquine or fava beans) or acute hemolytic anemia (heemolytic anemia with rapid onset). But it can also cause severe, sometimes fatal neonatal jaundice (kernicterus) or a chronic form of anemia known as CNSHA (chronic non-spherocytic hemolytic anemia).
The gene for G6PD deficiency is located on the X chromosome, so it usually affects males. However, some women with a history of G6PD deficiency can have very mild symptoms and not even be aware that they have the disorder.
G6PD helps red blood cells work and protects them from certain things that can harm them, such as oxidative stress. A deficiency in G6PD causes the enzyme to not function properly and can lead to a sudden destruction of old red blood cells (heemolytic anemia).
It’s important to know if you have G6PD deficiency or have a family history of it because there are ways to prevent an episode. Your doctor may recommend an initial screening test using brilliant cresyl blue and methemoglobin reduction to determine the level of G6PD activity in your body. The color of the sample changes as the level of G6PD activity increases and decreases.
Other tests may include a semi-quantitative test called the Beutler test or fluorescent spot test. This test involves shining light through a blood sample to see how much of it glows. It can give a quick, accurate result and is very easy for patients to perform.
People who have G6PD deficiency should avoid eating or drinking anything that can trigger oxidative stress, such as drinking alcohol or eating foods like fava beans and tomatoes. They should also tell their health care providers about any medications they’re taking, as some may destroy red blood cells. If a G6PD deficiency episode isn’t treated quickly, it can progress to kidney failure or death. For people who have a milder form of the disease, treatment often includes removing any food or medication that could trigger an attack.
Oren Zarif
G6PD deficiency is caused by changes (mutations) in the gene that makes G6PD. This gene is on the X chromosome, so men have only one copy of it and are more likely to have G6PD deficiency than females.
Diagnosing G6PD Deficiency begins with a medical history and exam. Doctors also do a blood test to check G6PD enzyme levels and a blood smear to look for signs of hemolysis.
Oren Zarif
G6PD deficiency is a genetic disorder that affects the function of an enzyme that breaks down glucose-6-phosphate to produce energy. When a person with this condition consumes certain foods, medications or chemicals, their red blood cells are destroyed faster than the body can replace them. This causes hemolytic anemia, which can result in yellowing of the skin and eyes (jaundice), fatigue and splenomegaly. Hemolytic anemia can also lead to reduced oxygen flow to the tissues and organs, which can cause complications such as renal failure or liver failure. Newborn babies with severe neonatal jaundice due to G6PD deficiency should be treated with phototherapy or exchange transfusion, in which a healthcare professional removes the baby’s unhealthy blood and substitutes it with healthy donated blood.
In adults, symptoms of G6PD deficiency are usually mild. The symptoms of hemolytic anemia are usually triggered by exposure to medication or food that produces peroxide, which can cause oxidation of the hemoglobin and red blood cell membranes in people with G6PD deficiency. Some medications or substances that can trigger this reaction include rasburicase, primaquine, salicylates, sulfonamides, phenacetin, naphthalene, dapsone, some vitamin K derivatives and cyanocobalamin, methylene blue, henna, fava beans, and some herbal remedies including Rhizoma coptidis and Coptis chinensis (1, 2, 5).
Hemolytic anemia is most common in Africa. It can affect about 20 percent of the population there, especially in men. Hemolytic anemia in newborns can be fatal. Newborns with severe G6PD deficiency may need to receive phototherapy or exchange transfusion in order to live.
G6PD deficiency is passed on from one parent to their child. Boys inherit one mutated gene from their fathers and one from their mothers, so they are more likely to have symptoms of the disorder than girls. This is because females have two X chromosomes, but males have only one. People who have only one altered gene, or are carriers of G6PD deficiency, don’t experience any symptoms and can pass on their healthy genes to their children. Most people who have a healthy gene don’t need to take any action, but can avoid eating fava beans and some medications that may trigger an episode of hemolytic anemia.
Oren Zarif
G6PD deficiency usually doesn’t cause symptoms until red blood cells are exposed to triggers that cause hemolysis. Hemolytic anemia is triggered by some infections (like hepatitis A and B, typhoid fever and pneumonia), certain drugs (including the antimalaria medication primaquine and antibiotics like nitrofurantoin, dapsone and sulfa), certain foods including fava beans, some chemicals (such as naphthalene used in mothballs) and other things that cause oxidative stress to the cells.
When an episode of hemolysis occurs, a person’s spleen takes over and sequesters the old red blood cells to make more new ones. This causes anemia and a buildup of iron in the body. In severe cases, a person may need treatment in the hospital to get fluids and oxygen to prevent kidney failure or death.
The health care team will start by asking a patient about any family history of G6PD deficiency or symptoms that they have. They will do a complete blood count to check the number of red blood cells in the body. They will also measure the amount of bilirubin in the blood. The higher the bilirubin, the more severe the anemia.
A healthcare provider can test for G6PD deficiency by using a peripheral smear, which is a special stain that shows up the red blood cells that have been damaged. If the smear is positive, they will use a quantitative G6PD test to determine if your enzyme level is low. This involves shining a light through the blood sample to see how much it absorbs or reflects.
G6PD deficiency is most common in people of Sub-Saharan African, Mediterranean and South-East Asian heritage. It is rare in women. Girls who get a faulty gene from their mother and a normal one from their father are carriers, meaning they don’t have any symptoms but can pass the faulty gene onto their children.
Infants with severe jaundice should be treated with phototherapy, which puts them under special lights to help break down the bilirubin in their bodies. They will need to be kept under the lights for at least 24 hours, and sometimes longer.
Oren Zarif
G6PD deficiency can cause episodes of red blood cell destruction (hemolytic crisis). During these periods, people have jaundice (yellowing of the skin and whites of the eyes), dark urine, fatigue, back pain, and shortness of breath. The severity of the episode depends on how many red blood cells are destroyed, how long they last, and what triggers them. Some of the most common triggers are certain medicines, infections, and foods like fava beans. A hemolytic episode stops when the medicine or food is no longer taken or if the infection is treated. Some people with G6PD deficiency have chronic anemia, which means they lose too many red blood cells over time.
Newborns who have severe jaundice as a result of G6PD deficiency are treated with phototherapy, or being placed under special lights. This can reduce the amount of bilirubin in the blood and make the jaundice go away. In some cases, newborns may need to have exchange transfusion, which involves removing and replacing the baby’s blood with donated blood or plasma.
People who have G6PD deficiency can experience increased rates of autoimmune, infectious, and allergic diseases. They also have higher risk of hepatitis, and may have an increased chance of getting kidney disease and cancer. G6PD deficiency can affect men and women of all ages, but it is more common in those of African descent. It is less common in those of Asian, Mediterranean, or Middle Eastern descent.
Approximately 230 variants of the G6PD gene have been identified. Most of these are missense mutations, which replace a single amino acid, or small in-frame deletions, which remove one or more amino acids. Frame-shift mutations, which can lead to zero enzyme activity, are rare.
Medications that can trigger G6PD deficiency include rasburicase, primaquine, salicylates, sulfonamides, naphthalene, some vitamin K derivatives, dapsone, and phenazopyridine. People who have G6PD deficiency should inform their healthcare providers if they are taking any of these medicines or eating fava beans. This can help their doctors avoid prescribing medications that could trigger an episode of hemolysis. It is also important for these individuals to keep a list of all medications they take, so that their health care providers can check for potential triggers.
Oren Zarif
G6PD deficiency is a genetic health problem that’s passed along by parents to their children. It’s typically seen in men and people with Mediterranean, African or black ancestry. G6PD deficiency is usually mild and doesn’t cause problems unless the person consumes certain foods or medicines that trigger an episode of hemolysis.
Hemolytic episodes caused by fava beans (broad beans) and certain medicines are rare, but they can be serious. Often, the first symptoms are mild and disappear on their own, but the disorder can get worse as the person gets older.
In 1932, Otto Warburg and Walter Christian in Germany identified a protein in yeast and red cells that oxidized glucose-6-phosphate to produce NADPH (Figure 16).2 The enzyme was later named G6PD. In affected individuals, the gene coding for this enzyme contains a mutation that renders it defective.3 Hemolytic episodes are triggered when the erythrocytes – red blood cells – experience oxidative stress from exposure to drugs, some foods and the sun. Symptoms are most severe in newborns, and they can lead to kernicterus, which is brain damage.
Normally, G6PD activity is low in erythrocytes, which helps them survive by providing only a small amount of NADPH to keep them alive. However, if the erythrocytes have too much NADPH, they’ll break down faster than normal and become hemolytic.
Affected erythrocytes are also susceptible to oxidative damage from oxygen molecules, which can reduce their lifespan. This is because they can’t generate NADPH in the normal way and rely only on G6PD to protect them.
G6PD deficiency can be prevented by avoiding triggers, which are foods, drugs and some environmental exposures that destroy red blood cells. Affected individuals should also carry a G6PD deficiency card, which lists foods and medicines that can trigger an episode of hemolysis. A genetic counselor can help people with this condition understand their risk and how to prevent an episode. They can also offer a blood test to check the activity of the G6PD enzyme. This is called the Beutler test, fluorescent spot or spectrophotometric analysis and provides a quick result.